INTRODUCTION:
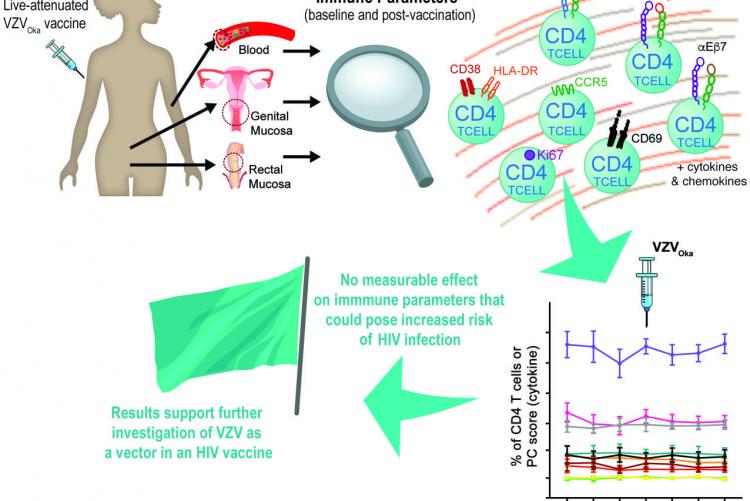
A protective HIV vaccine would be expected to induce durable effector immune responses at the mucosa, restricting HIV infection at its portal of entry. We hypothesise that use of varicella-zoster virus (VZV) as an HIV delivery vector could generate sustained and robust tissue-based immunity against HIV antigens to provide long-term protection against HIV. Given that HIV uniquely targets immune-activated T cells, the development of human vaccines against HIV must also involve a specific examination of the safety of the vector. Thus, we aim to evaluate the effects of VZV vaccination on the recipients' immune activation state, and on VZV-specific circulating humoral and cellular responses in addition to those at the cervical and rectal mucosa.
Perciani CT1, Jaoko W2,3, Walmsley S4, Farah B2, Mahmud SM5, Ostrowski M1,6, Anzala O2,3, Team KI2, MacDonald KS1,7.
METHODS AND ANALYSIS:
This open-label, randomised, longitudinal crossover study includes healthy Kenyan VZV-seropositive women at low risk for HIV infection. Participants receive a single dose of a commercial live-attenuated VZVOka vaccine at either week 0 (n=22) or at week 12 (n=22) of the study and are followed for 48 and 36 weeks postvaccination, respectively. The primary outcome is the change on cervical CD4+ T-cell immune activation measured by the coexpression of CD38 and HLA-DR 12 weeks postvaccination compared with the baseline (prevaccination). Secondary analyses include postvaccination changes in VZV-specific mucosal and systemic humoral and cellular immune responses, changes in cytokine and chemokine measures, study acceptability and feasibility of mucosal sampling and a longitudinal assessment of the bacterial community composition of the mucosa.
ETHICS AND DISSEMINATION:
The study has ethical approval from Kenyatta National Hospital/University of Nairobi Ethics and Research Committee, the University of Toronto Research Ethics Board and by Kenyan Pharmacy and Poisons Board. Results will be presented at conferences, disseminated to participants and stakeholders as well as published in peer-reviewed journals.
TRIAL REGISTRATION NUMBER:
NCT02514018. Pre-results.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
KEYWORDS:
HIV vaccine; herpes zoster; herpesviridae infections; immune activation; mucosal immunity; varicella-zoster virus; virus diseases